Chapter 12: Amines
Important Questions
1. Arrange the following compounds in an increasing order of their solubility in water:
C6H5NH2, (C2H5)2NH, C2H5NH2
2. Write the structure of 2-aminotoluene.
3. Account for the following:
(i) Primary amines (R − NH )2 have higher boiling point than tertiary amines R N 3 ( )
(ii) Aniline does not undergo Friedel-Crafts reaction.
(iii) (CH3)2NH is more basic than (CH3)2 in an aqueous solution.
4. State reasons for the following:
(i) pKb value for aniline is more than that for methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not soluble in water.
(iii) Primary amines have higher boiling points than tertiary amines.
5. (a) Explain why an alkylamine is more basic than ammonia?
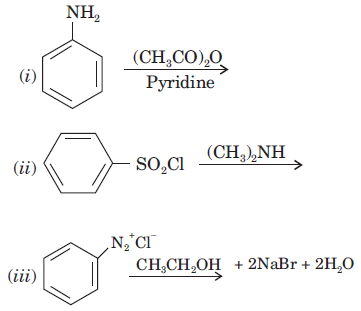
(b) How would you convert?
(i) Aniline to nitrobenzene
(ii) Aniline to iodobenzene