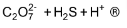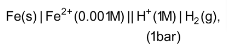Important Questions
1. The chemistry of corrosion of iron is essentially an electro-chemical phenomenon. Explain the reactions occurring during the corrosion of iron in the atmosphere.
2. Complete the following chemical equations:
(i) MnO4– + C2O42– + H+
(ii) 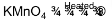 (iii)
(iii) 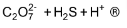
3. Calculate the emf of the following cell at 298 K:
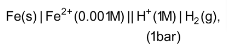
Given E0cell = +0.44V)
4. Express the relation between conductivity and molar conductivity of a solution held in a cell.
5. The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm–1. Calculate its molar conductivity.
Sample Questions
1. Define the following terms:
(i) Molar conductivity (Λm)
(ii) Secondary batteries
2. Conductivity of 0.00241 M acetic acid solution is 7.896 × 10–5 S cm–1. Calculate its molar conductivity in this solution. If Δ0m for acetic acid be 390.5 S cm2 mol–1, what would be its dissociation constant?
3. What type of a battery is lead storage battery? Write the anode and the cathode reactions and the overall reactions occurring in a lead storage battery.
4. Calculate the time to deposit 1.5 g of silver at cathode when a current of 1.5 was passed through the solution of AgNO3. (Molar mass of
Ag = 108 g mol–1, 1F = 96500 C mol–1)
5. What type of cell is a lead storage battery? Write the anode and the cathode reactions and the overall cell reaction occurring in the use of a lead storage battery?
OR
Construct the redox equation from the two half cell reactions and predict if this reaction favours formation of reactants or product shown in the equation.
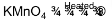 (iii)
(iii)