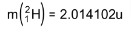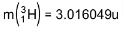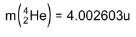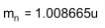Important Questions
1. Define the activity of a given radioactive substance. Write its S.I. units.
2. Why is it found experimentally difficult to detect neutrinos in nuclear β-decay?
3. State the law of radioactive decay. Plot a graph showing the number (N) of undecayed nuclei as a function of time (t) for a given radioactive sample having half life. Depict in the plot the number of undecayed nuclei at (i) t = 3T1/2 and (ii) t = 5T1/2
4. Draw a plot of potential energy between a pair of nucleons as a function of their separation. Mark the regions where potential energy is (i) positive and (ii) negative
5. (a) Derive the mathematical expression for law of radioactive decay for a sample of a radioactive nucleus.
(b) How is the mean life of a given radioactive nucleus related to the decay constant?
Sample Questions
1. A radioactive isotope has a half-life of 10years. How long will it take for the activity to reduce to 3.125%
2. Four nuclei of an element undergo fusion to form a heavier nucleus, with release of energy. Which of the two: the parent or the daughter nucleus would have higher binding energy per nucleon?
3. A heavy nucleus X of mass number 240 and binding energy per nucleon 7.6 MeV is split into two fragments Y and Z of mass numbers 110 and 130 respectively. The binding energy of nucleons in Y and Z is 8.5 MeV per nucleon. Calculate the energy Q released per fission in MeV.
4. Distinguish between nuclear fission and fusion. Show how in both these processes energy is released. Calculate the energy release in MeV in the deuterium-tritium fusion reaction:

Using the data:
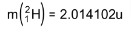
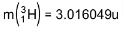
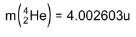
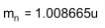
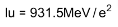
5. Explain the processes of nuclear fission and nuclear fusion by using the plot of binding energy per nucleon (BE/E) versus the mass number A.